Seed Oils as a Driver of Heart Disease
Highlights
The United States saw a catastrophic rise in heart disease during the mid-20th century most likely related to changes in dietary linoleic acid intake.
Researchers found a significant disparity in the incidence and prevalence of heart disease, with cultures eating pre-industrial diets having extremely low incidence, if any, compared to populations eating industrialized diets.
Before any human trials had been conducted, leading public health organizations assumed that lowering serum cholesterol levels and increasing seed oil consumption would lead to better health outcomes.
In the United States, steadily rising seed oil consumption drove linoleic acid intake up from 2% of calories in the early 1900s to around 8.9-9.4% by 2010.
By the late 20th century, research had revealed that the susceptibility of LDL particles to oxidation was a much better predictor of heart disease than changes in total cholesterol or LDL cholesterol levels.
Numerous human clinical trials demonstrate that, like smoking, increased seed oil consumption increases LDL’s susceptibility to oxidation, posing a major concern when it comes to cardiovascular disease.
Like smoking, seed oil consumption also induces oxidative stress, induces insulin resistance, damages the arterial endothelial wall, and increases oxidized LDL – a common mechanism that causes heart disease.
Introduction
In 1955, President Dwight D. Eisenhower suffered his first heart attack at the age of 64.
While heart disease rates were climbing throughout the 20th century [*], the President’s heart attack put the problem of heart disease squarely on the map in the minds of most Americans.
After all, Eisenhower had no family history of heart disease and no obvious risk factors after he quit smoking in 1949. He exercised regularly, and his weight was considered optimal. His blood pressure was well controlled, and his total cholesterol was in the safe range, according to cardiologists, at 165 mg/dl [*].
Over the span of six weeks, as Eisenhower recovered from his heart attack, the public learned about his progress via twice-daily press conferences. This didn’t just keep Americans appraised of their leader’s health but also gave everyone a crash course on heart disease at a time when strokes and heart attacks were on the rise.
What came next shaped how most people dealt with heart disease for the next 60 years.
Eisenhower pursued very public dietary changes that included eating a low-fat diet. When he did use fat, it was soybean oil for cooking and seed oil-based margarine to keep cholesterol levels low.
Unfortunately, the more he dieted and consumed seed oils, the more his health declined — all of which was being documented by his doctors.
In March 1959, Eisenhower took his diet even further, adopting a high corn oil diet. Still, he and his doctors grew increasingly frustrated as his health continued to decline. He died of heart disease just 10 years later at age 78 [*].
It’s worth noting that Eisenhower had embraced a low-fat, high vegetable oil diet before randomized-controlled trials (RCTs) had ever tested the hypothesis that such a diet could stem the rising tide of heart disease [*].
Still, did Eisenhower’s high corn oil diet and renunciation of all other fats help or hurt his health? And how did Eisenhower’s battle with heart disease and subsequent dietary changes help spur a rise in seed oil consumption in the U.S. and beyond?
Heart Disease, Once Rare, Becomes Common

President Eisenhower was far from alone in his struggle with heart health. Heart disease was on the rise, with the proportion of Americans dying from heart disease nearly tripling between 1900 to 1960 [*].
Paul Dudley White, the “father of American cardiology” [*], gained national prominence when he consulted with President Eisenhower’s medical team during the President’s myocardial infarction in 1955 [*].
And in 1971, Dr. White wrote a short retrospective on his then 60-year career in medicine, noting that when he began to practice, heart disease was rare and that heart attacks were virtually unknown [*].

Other physicians noted the sparse number of references to heart attacks in early 20th-century literature. They also noted that while “coronary heart disease” as we know it was first described in 1912, it was not added to the international registry of death codes until 1930, suggesting a lack of need [*].
There are different estimates for the rise in heart attacks and heart disease-related deaths, but they all show this steep increase through the 1960s [*,*].
Many more physicians commented on the increase in heart disease.
“[James] Mackenzie, with all his earlier experience in general practice and later as a leading consultant in cardiology, wrote (as late as 1923) that [only] 380 patients had consulted him for angina pectoris [a type of chest pain caused by reduced blood flow to the heart]. Today (1946), as [Maurice] Cassidy remarks, ‘the modern cardiologist's name is legion’ and ‘he counts his cases by thousands rather than hundreds.’” [*]
Not only was heart disease much more common in the middle of the 20th century than in any other time in history, but it seemed to be affecting people at an earlier age. For instance, sons predeceased their fathers in one notable 1962 study, leading the study authors to wonder:
“What changes in environment may have taken place during that quarter of a century? One wonders whether a change in diet occurred or whether an increase in the tension of modern living, or some more specific cause, is behind all this.” [*]
Physicians in the United States, Great Britain, and other countries [*] speculated about what was behind the steep increase in heart disease cases and related deaths, and came to conclusions similar to those operant today — that heart disease is caused by smoking, obesity, diabetes, etc.
Early attempts to determine the relative increase in heart disease were muddled by data and diagnostic issues. Later attempts produced a clearer picture, once the disease was better characterized.
In one of the first efforts to clarify the catastrophic rise in heart disease, researchers conducted a detailed analysis looking at the geographic spread of the disease.
Using the gold-standard method of autopsies for diagnosing heart disease, the authors determined that varying populations living in the U.S. had much higher rates of heart disease than those living in their native countries. And in some of those countries, heart disease-related incidents were effectively zero [*].

Of the Japanese-Americans (blue) who were autopsied, 18.7% had suffered a heart attack at some point in their life compared to only 3.1% of Japanese (orange). Additionally, 12.7% of the African-Americans (blue) autopsied had suffered a heart attack at some point in their life compared to only 0.1% of Africans (orange).
In addition, an analysis of the worldwide literature from 1955 to 1968 found a wide variation in the incidence and prevalence of heart disease, with cultures eating traditional, pre-industrial diets having extremely low incidence, if any [*,*,*].
While eliminating genetics as a cause of heart disease, those authors couldn’t identify exactly what environmental factor was driving the increase.
Seed Oils Proposed as the Cure For Heart Disease
One of the most well-known hypotheses for the rise in heart disease in the 20th century was that certain fats (namely saturated fats) were to blame and that replacing them with polyunsaturated fats (the primary fat in most seed oils) would be healthier.
And it all started when researchers found some correlations between dietary fat intake and blood cholesterol levels.
Initially, it seemed to make sense — doctors and researchers found that diseased arteries contained atherosclerotic plaques, which are partly composed of cholesterol. Arterial plaques are like scars — they are the body’s attempt to repair damaged spots on the arterial walls. While these “scars” aren't inherently bad, more arterial damage equates to more plaques, i.e. more cholesterol in the blood. Naturally, doctors and researchers surmised that higher blood cholesterol levels increased the odds of heart disease.
When polyunsaturated fats, like those found in seed oils, were found to lower serum cholesterol levels, researchers assumed that lower cholesterol levels from this dietary change would lead to better heart health outcomes.
On this basis, in 1961, the American Heart Association (AHA) began recommending seed oils high in polyunsaturated fats as a “possible means of preventing atherosclerosis and decreasing the risk of heart attacks and strokes [*].” At this point, no human trials had been conducted on the efficacy of this therapy, but one was underway — the “Anti-Coronary Club Study,” which was mentioned in the AHA paper [*].
What Are Seed Oils?
Seed oils are vegetable oils produced from seeds, such as corn, cottonseed, soybeans, etc., as opposed to oils pressed from fruits, like olive and avocado oil.
Seed oils tend to be low in saturated fats and high in omega-6 polyunsaturated fats (PUFA), specifically linoleic acid (LA). Seed oils also often contain some beneficial monounsaturated fat and some omega-3 PUFAs, typically alpha-linolenic acid (ALA).
Both types of PUFAs are unstable and tend to oxidize or go rancid quickly. Rancid omega-3 fats are what you smell when a fish goes bad and are the reason omega-3 fats are not generally used in processed foods, as the rotten fish smell is unappealing as well as toxic.
Linoleic acid is also a precursor for inflammatory molecules. This is an important reason why, in excess, linoleic acid can contribute to pathological inflammation and tissue damage [*,*]. Conversely, the omega-3 fats alpha-linolenic acid and docosahexaenoic acid (DHA) are precursors for anti-inflammatory molecules.
Throughout our evolutionary history, our diets contained about a 1:1 ratio of omega-6 to omega-3 [*]. However, today that ratio in the U.S. is between 10:1 to 20:1, and as high as 50:1 in some parts of the world, such as urban India [*,*]. That means we’re eating 10, 20, or 50 grams of omega-6 fat for every one gram of omega-3 fat. And this drastic shift in fatty acid intake came from the increase in dietary omega-6 fats, specifically linoleic acid from seed oils.
For context, in 1909, linoleic acid comprised ~ 2.79% of total caloric intake in the United States [*]. By 1999 it had increased to ~ 7.21%, and by 2010 it was around 8.9-9.4% [*].
As the importance of PUFA intake wasn’t discovered until the late 1920s [*], and the “omega” notation for PUFA wasn’t introduced until 1964 [*], distinctions between the different types of PUFAs were not made in early research and data gathering. So while it is possible to estimate consumption, direct measurements were not made [*]. Additionally, no formal data collection of foods that were available or consumed was conducted before 1909.
However, we do know that in 1909, meat, poultry, and fish contributed 29.4% to people’s total PUFA intake [*]. And their total intake was relatively low at 13 grams per day. Fats and oils contributed about a third [*].
Testing The Seed Oil Hypothesis
By 1965, results of the first human trial testing the effects of linoleic acid-rich corn oil on cholesterol levels and heart disease were published. The Rose Corn Oil trial reported [*]:
“At two years, the proportion of patients remaining free of major cardiac events is greater for the control group (75%) than for the two oil groups (olive oil 57%, corn oil 52%).”
Corn oil is high in linoleic acid [*], which is why it was used in this trial, but the linoleic acid level of olive oil was not reported. But, typically, olive oil is much lower in linoleic acid than corn oil. The omega-6 content was not directly tested for any of the three groups.
The corn oil group did have lower cholesterol levels, but they also suffered more heart attacks and deaths. The authors concluded, “under the circumstances of this trial corn oil cannot be recommended in the treatment of ischaemic heart disease [*].”
The following year, the report of the Anti-Coronary Club trial was released [*], which was the only trial mentioned by the 1961 AHA position statement to test the hypothesis that increased seed oils would reduce heart disease in humans.
The intervention was to decrease the amount of saturated fat and increase the amount of polyunsaturated fat. They did this by replacing the consumption of beef, butter, etc., with “high [polyunsaturated/saturated] ratio margarines and a minimum of one ounce of vegetable oil daily."
This study did have a number of methodological problems, including the absence of a proper control group. Among other issues, smoking was not recognized as a heart-disease risk factor in 1959, so when the control group was added to the study, smokers were almost twice as prevalent in the control group.
“At the initiation of the study, smoking was not stressed as a CHD risk factor. Our data indicated that there were significantly more smokers at entry in the control group than in the active experimental group at both age levels. In the 50 to 59-year-old group, 17.7% of the active experimental group and 36.0% of the control group smoked…. In the younger age category 25.9% of the active experimental group smoked compared to 44.3% of the control group...” [*].
Nevertheless, while more members of the control group had cardiac events, none died from them, unlike the experimental group. Even the authors note that, “[the results do] appear somewhat unusual…” Also, fewer control members died from non-cardiac causes than in the experimental group, although the difference was small.
As a strict test of smoking versus seed oil consumption, one would have to conclude smoking was less harmful. However, the relative reduction in cardiac events was considered to be a triumph, and the “prudent diet” of increased omega-6 consumption became a mainstay of cardiovascular recommendations.
Despite a lack of evidence for efficacy in preventing death from heart disease, physicians and vendors of seed oils charged along in recommending consumption of polyunsaturated seed oils.

CPC International Inc.’s 1970s campaign for corn oil. CPC is now known as Best Foods [*].
Still, the authors of the 1961 AHA advisory recognized the need for a trial that would, once and for all, prove the efficacy of linoleic acid for the prevention of heart disease.
In this new study, most participants were to live at home, but the most “controlled” part of the study would be conducted in a hospital environment. It became known as the Minnesota Coronary Experiment (MCE).
“...a well-controlled mass field trial was needed to test the hypothesis that, among middle-aged American men, alteration of the amount and type of fat and the amount of cholesterol in the diet would decrease the incidence of future clinical coronary heart disease.” [*]
While the MCE trial finished in 1972, the report was not issued until 1989, under the signature of only one of the two principal investigators [*]. The final results weren’t published until National Institute of Health researchers, attempting to conduct a meta-analysis of linoleic acid and heart disease, re-analyzed the results using previously unpublished data [*].
They found that while increased linoleic acid did reduce serum cholesterol levels, this was associated with the “possibility” of a higher rate of death and no benefit.
“This finding that greater lowering of serum cholesterol was associated with a higher rather than a lower risk of death in the MCE does not provide support for the traditional diet-heart hypothesis.”
In other words, the process of proving the 1961 AHA hypothesis that omega-6 fats could lower the risk of heart disease-related death that began with the ill-advised 1961 AHA advisory had been completed, and the hypothesis was disproved.
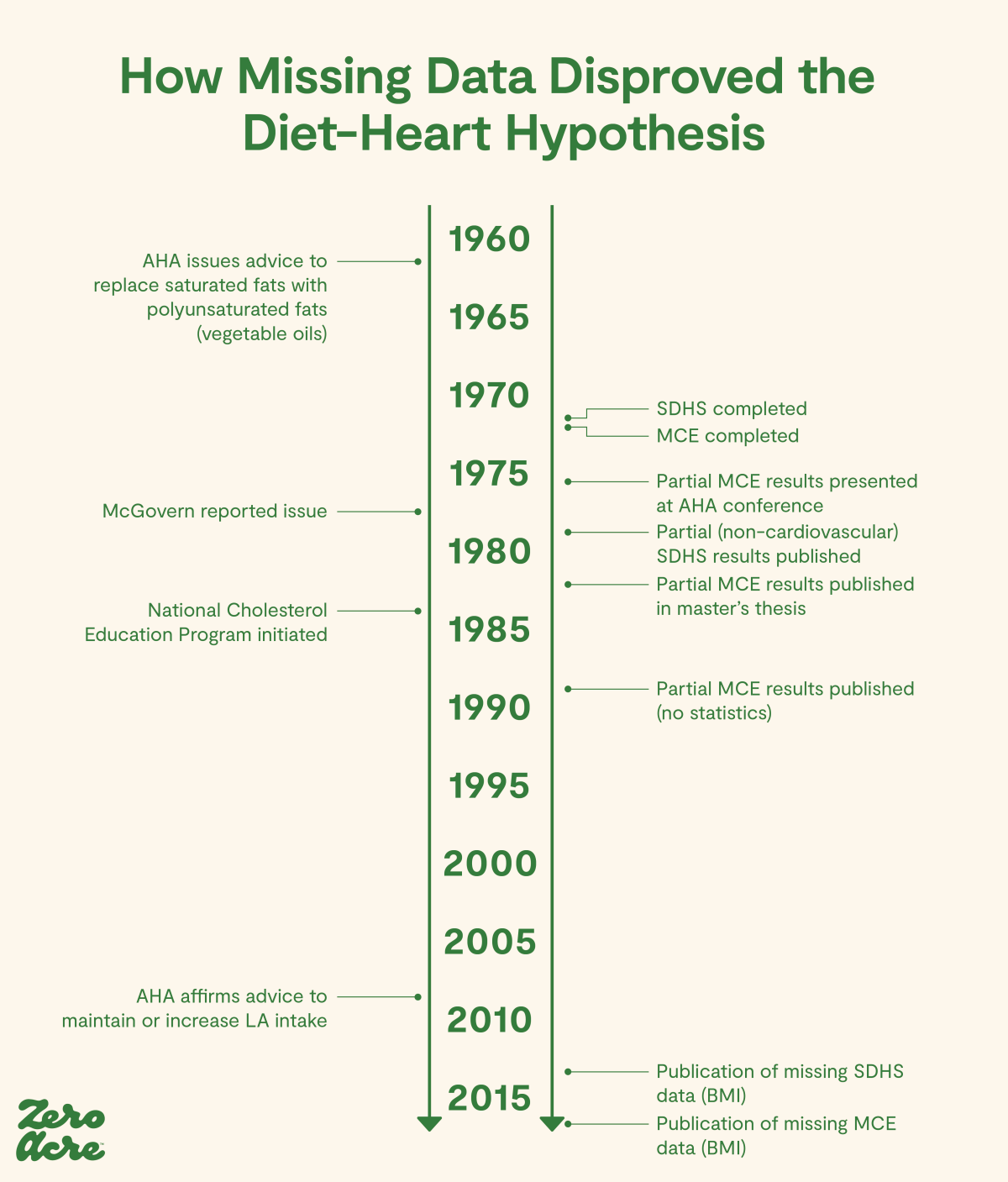
Timeline adapted from [*].
In 2014, one of the leading heart disease researchers in the world (who also discovered that trans fats and oxidized cholesterol induce heart disease), summarized the evidence linking seed oils to heart disease as follows:
“No clinical trial has succeeded in lowering the risk of CVD [cardiovascular disease] using an increased intake of [seed oils].” [*]
A meta-analysis of available randomized-controlled trials (RCTs) also concluded that:
“[...] replacement of saturated fat with [seed oils] effectively lowers serum cholesterol, but does not support the hypothesis that this translates to lower risk of death from coronary heart disease or all-causes.” [*]
So, there was never evidence that seed oils, or increased intake of omega-6 PUFAs in place of other fats, lowered the risk of heart disease.
But that wasn’t enough to convince some researchers that high-linoleic seed oil couldn’t stop heart disease in its tracks. The AHA continues to recommend it to this day [*].
The Widespread Introduction of Seed Oils
It’s often thought that the introduction of seed oils into the diet started with the introduction of products like Crisco in the market in the early 20th century (Crisco was brought to market in 1911) [*].
But the industrial production of cottonseed oil in the U.S. began long before, as manufacturers looked for something to do with the oily byproduct of cotton processing.
Due to the oil’s toxicity (including producing permanent infertility in men [*], among other effects), it was not suitable for human consumption unless it could be made less toxic [*].
Nevertheless, cottonseed oil was widely used as an adulterant in lard produced for food in the United States [*] and exported worldwide in the late 1800s [*]. Due to the criminal nature of this activity, and the general lack of statistics, exact distribution and consumption numbers aren’t available.
However, even partially hydrogenated fats such as Crisco have higher amounts of linoleic acid than traditional fats, such as butter or tallow [*]. Such a switch would have increased linoleic acid consumption.
Modern attempts to reconstruct our consumption of linoleic acid, the active component in seed oils, show that consumption increased steadily during the 20th century from 2% of caloric intake to 8% [*,*], primarily from soybean oil.
In the U.S., this increase was concurrent with the emergence of heart disease (and other chronic diseases) as a growing cause of death and morbidity [*,*,*,*].
Since omega-6 fats are largely found in vegetable fats such as seed oils, it’s useful to look at the increasing intake of vegetable fats over the early part of the 20th century.
As the AHA noted in 1961, “Considerable amounts of saturated fat are present in whole milk, cream, butter, cheese, and meat…. In contrast to the above food fats, many natural vegetable oils, such as corn, cotton, and soya, as well as the fat of fish, are relatively low in saturated fats and high in fats of the polyunsaturated type.” Thus some papers refer to animal fats interchangeably with saturated fats. Although technically incorrect, we will follow the AHA’s lead, and refer to these as saturated fats.

Adapted from study figure 3. Sources of nutrient fat per capita/day [*].
Due to the rise of seed oils throughout the 20th century, in 2010, fats and oils were responsible for a whopping three-quarters of the PUFAs people consumed. Americans were now consuming 44 grams of PUFAs per day, more than triple the amount from 1909. The types of PUFAs also changed, as the increase in seed oils biased consumption towards omega-6 fats and away from omega-3 fats [*].
The Rise in Seed Oils Parallels the Rise in Heart Disease
The notion that an increase in linoleic acid would protect against heart attacks was a curious one, as the biggest change in Americans’ diets during the period in which heart attacks became common was an increase in high linoleic seed oil consumption.
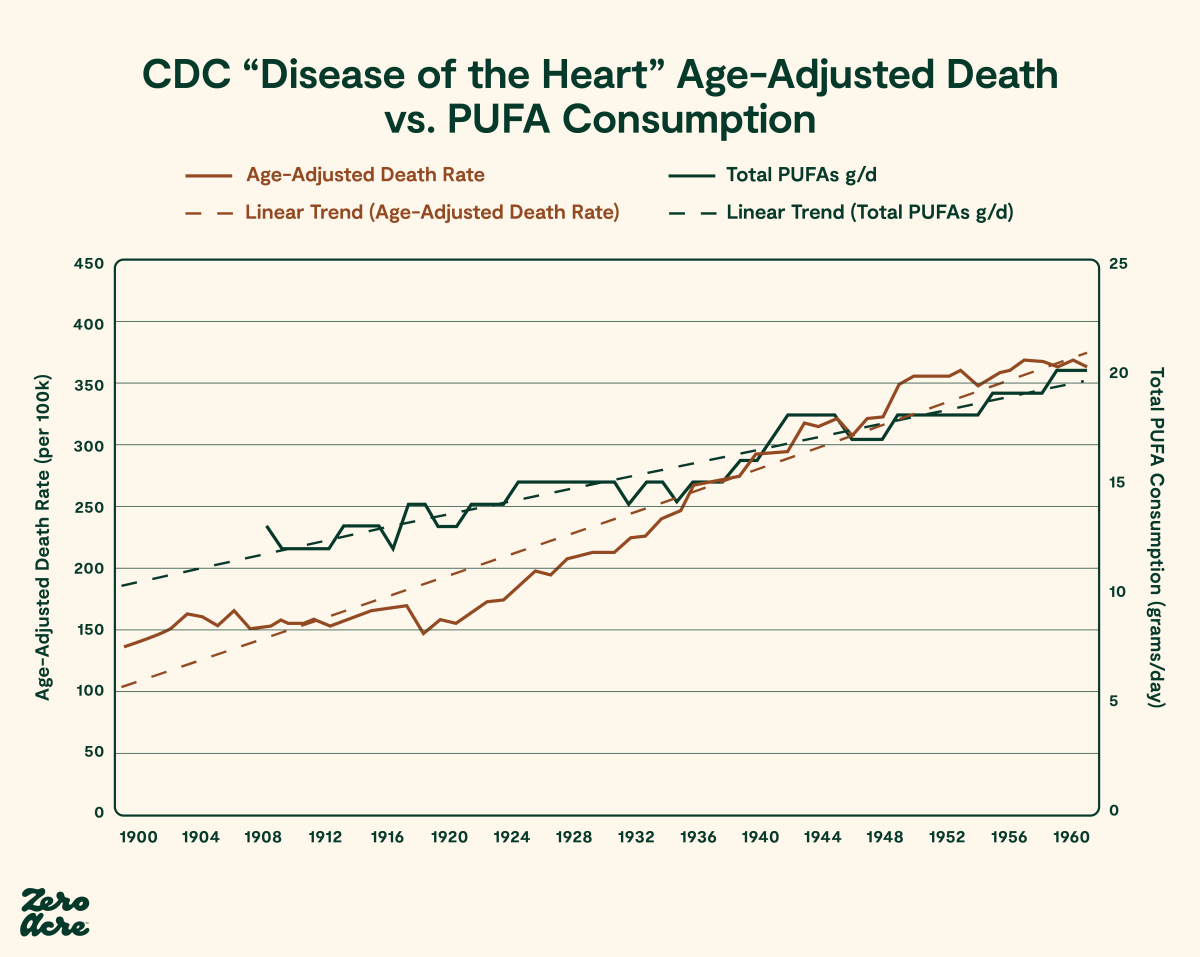
Age-adjusted deaths from heart disease per 100,000 people are plotted on the left vertical axis [*]. Loss-adjusted food availability per capita for total PUFA in grams per day is plotted on the right vertical axis [*]. The time period from 1900 to 1962 in the United States is plotted on the bottom horizontal axis.
After 1961, deaths from heart disease started declining fast. This was because many people stopped smoking after the public was informed of its association with heart disease and cancer. Having fewer smokers was a major win in the battle against heart disease, but to this day, the incidence of heart disease remains higher than it was in 1900. This means other factors are still playing a devastating role.
It's helpful to look to other countries to verify these trends. In fact, these aren't unique to the U.S. By 1996, people living in London and Israel consumed 6-11% and 12% of their total calories from linoleic acid, respectively [*,*,*].
Indeed, global vegetable oil production has increased by over 1600% since 1909, has doubled in the last 20 years, and is expected to grow 30% in the next four years [*]. Additionally, U.S. consumption of soybean oil alone (55% linoleic acid) has grown more than 1000-fold since 1909 [*,*].

Assuming the rise in dietary linoleic acid from seed oil consumption continues as it has been, the United States may be closer to 13% in 2022. And one way we can estimate the rise in consumption (and its subsequent effect on our health as a population) is via linoleic acid concentration in fat tissue.
Linoleic acid concentration in fat tissue increased ~ 2.5-fold between 1959 and 2008, going from 9.1% to 21.5% [*].
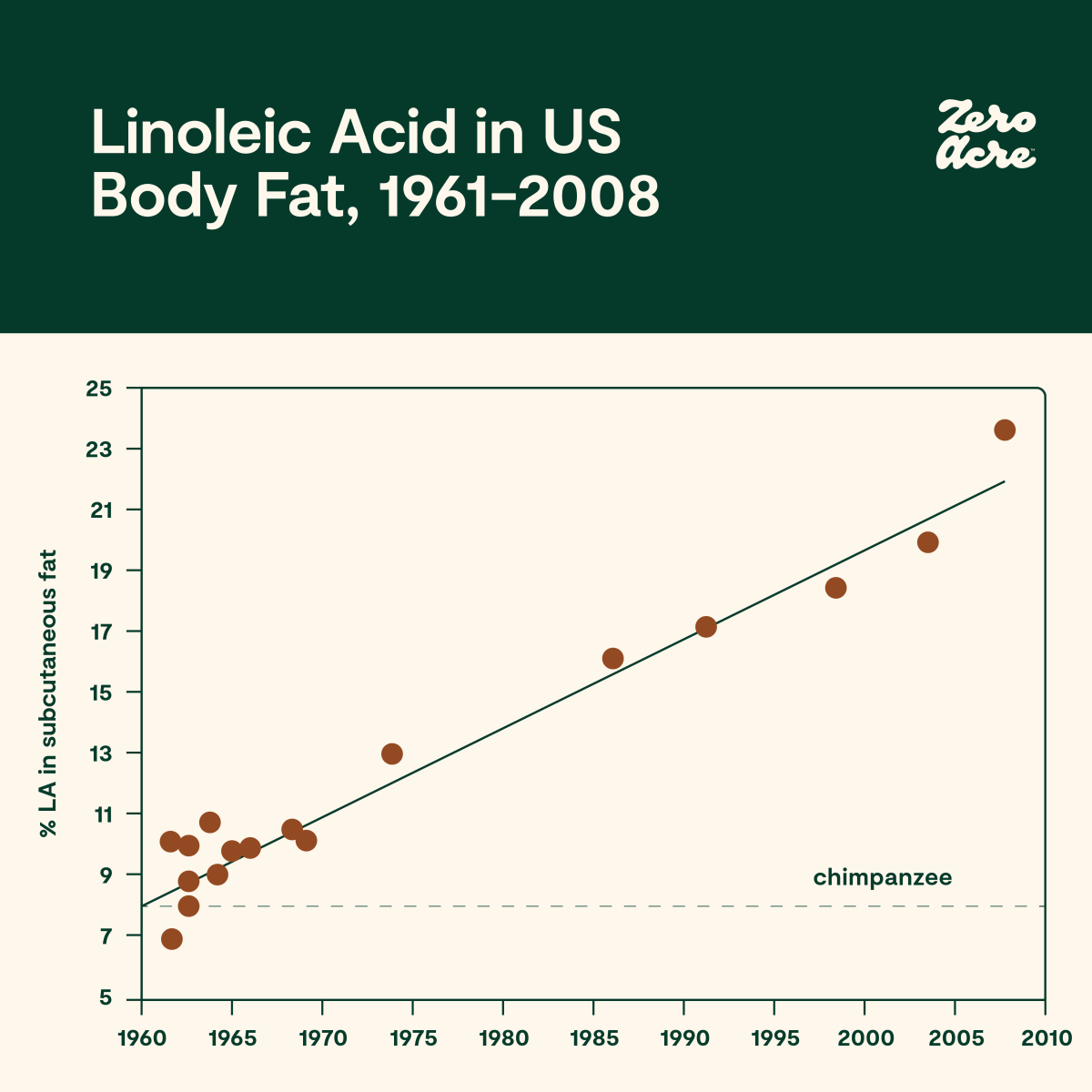
Study figure 1A. Linoleic acid concentration across all subcutaneous adipose tissue sites in the United States from 1959 to 2008 [*].
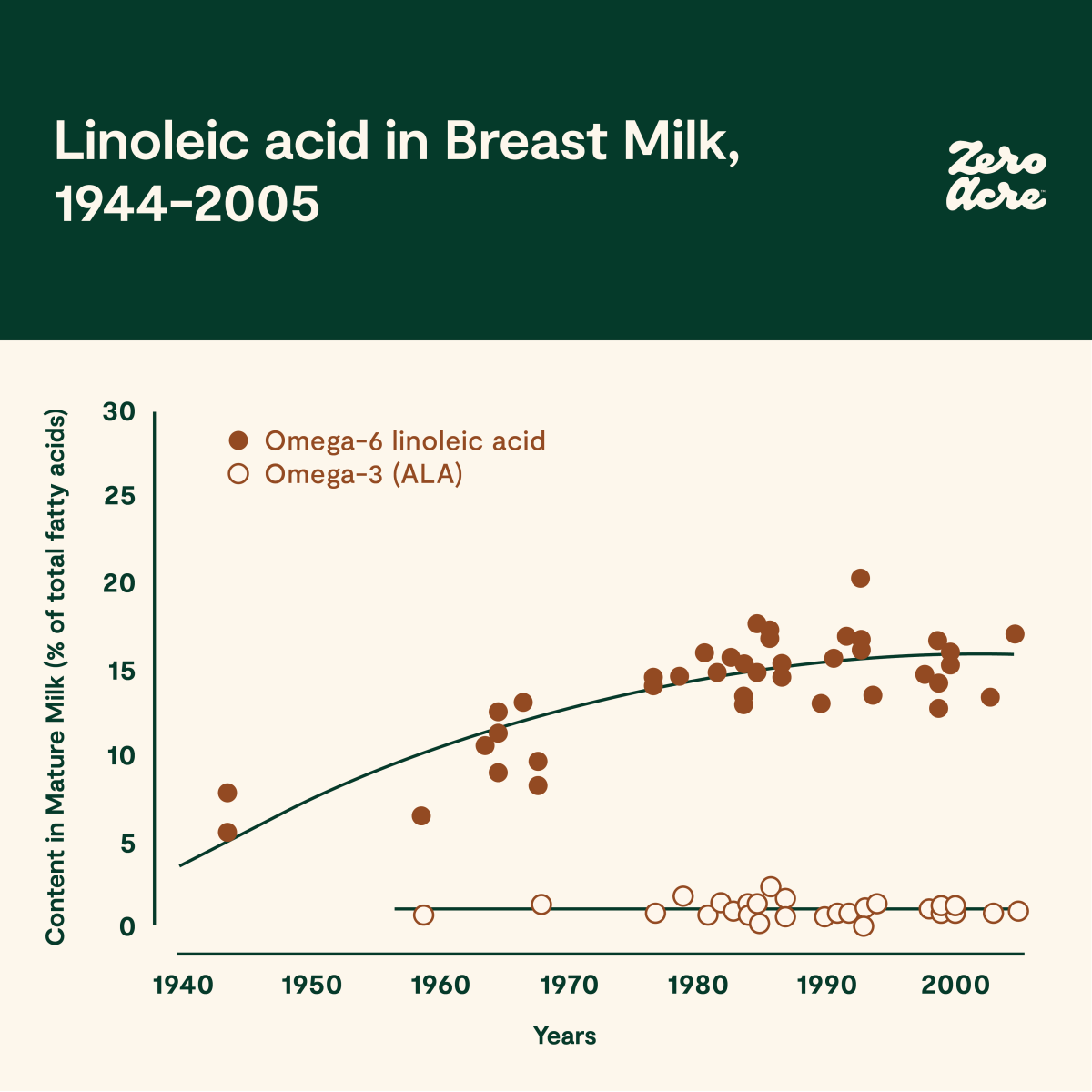
Study figure 4. Concentration of linoleic acid as a percentage of total fatty acids in mature breast milk of women living in the United States and the United Kingdom from 1944 to 2005 [*].
Seed oil consumption estimates, production figures, and measurements taken from fat tissue and breast milk, all point to omega-6 linoleic acid becoming a major source of calories in industrialized diets.
What Do Populations With Low Rates of Heart Disease Eat?
If the modern diet, with its unprecedented increase in linoleic acid consumption, is linked with a rise in heart disease, then what are populations with low rates of heart disease eating?
Modern hunter-gatherer populations are free of chronic diseases like obesity and heart disease, whether their traditional diets are high in fat or carbohydrates. This is reflected in comparisons made between Africans and Asians that moved to the United States, while their counterparts stayed in their countries of origin [*].
In countries eating industrial diets, there are individuals who eat seed oils and don't get heart disease, just like there are smokers who don’t get lung cancer. However, all populations with low rates of heart disease don't eat seed oils in significant quantities.
One example includes the Tsimane, an Amazonian tribe renowned in the cardiology world as having “the lowest reported levels of coronary artery disease of any population recorded to date [*,*,*].” While the Tsimane population is just starting to eat seed oils, their level of consumption is about the same as the U.S. before its heart disease epidemic started to skyrocket in the mid-20th century.

BBC article reporting on the Lancet’s study findings about the Tsimané’s unmatched heart health [*].
The Kitavans, a South Pacific tribe intensively studied in the 1990s, were similarly healthy, with the late anthropologist and author Staffan Lindeberg reporting that “stroke and ischaemic heart disease (IHD) appear to be absent…” within the population [*,*]. At the time, the Kitavans depended on a diet primarily of fish, coconut, and tubers and virtually free of seed oils or margarine.
And these populations weren’t without risk factors for heart disease. Lindeberg also reported that “75% of [Kitavan] men and 80% of [Kitavan] women are daily smokers, mainly of imported black or home-grown tobacco [*].”
Yet, tobacco use didn’t seem to increase disease rates in the Kitavan population. We don't know exactly why, as these are only observations, not experiments. However, one possibility is that Kitavans smoked tobacco leaf, not cigarettes like industrialized populations.
Cigarettes are “easier” to smoke because of additives like sugar, while pure tobacco leaf is much harsher, which may make it more self-limiting. Cigarettes also contain toxic additives like formaldehyde, lead, arsenic, ammonia, and hydrogen cyanide, to name a few [*].
This difference in what exactly both populations were smoking may partially explain why Kitavans don’t suffer from more heart disease. The other reason, however, is most likely due to their lifestyle, which is healthier overall than that of industrialized populations. In other words, cigarette smoking alone may not be enough to cause the epidemic levels of heart disease-related deaths seen in the United States in the absence of other risk factors.
For example, Kitavans get substantially more beneficial sun exposure than most people in the Western world. More regular, healthy sun exposure is just as important in reducing mortality risk as transitioning from being a smoker to a non-smoker [*].
The Japanese are another population we can look to in order to understand the effects of dietary transitions on cardiovascular health. The Japanese underwent major dietary transitions after World War II under American occupation — namely, a dramatic increase in seed oil consumption.
Okinawa, the southernmost major Japanese island and a large U.S. military base, saw the fastest dietary shifts. Okinawans were formerly renowned for their longevity, but younger Okinawans, consuming a diet conforming to U.S. patterns, suddenly found themselves dying faster than any other population in Japan [*].
Japanese scientists were among the first to point out the dangers of “excess” linoleic acid from seed oils, saying that there is “no rational, empirical basis for increasing linoleic acid intake to current levels” (i.e., > 6% of energy in Japan and the United States) [*].
The Real Role of LDL in Heart Disease
At this point, we’ve covered that early trials on increased seed oil consumption showed a decrease in LDL (low-density lipoprotein) cholesterol levels, but didn’t seem to improve disease or mortality rates.
And there’s clear evidence that populations with low seed oil consumption seem to fare better health-wise than those with higher intakes.
But what does the science say today after decades of connecting cholesterol levels (especially LDL) with heart disease? Is LDL involved with heart disease at all? And if so, then how?
Omega-6 fats do decrease total cholesterol and low-density lipoprotein (LDL) levels compared to some more saturated fat sources.
And the lower the LDL cholesterol (LDL-c) or LDL particles (apo-b), the lower the risk of cardiovascular disease according to conventional cardiology.
However, research in the 1980s and ‘90s revealed that changes in total cholesterol or LDL cholesterol levels were not good predictors of who got heart disease. Instead, a much better predictor was the susceptibility of LDL particles to oxidation [*,*]. Oxidation is the chemical process that rusts metal or causes the wax in a candle to burn. It also takes place in every cell of your body.
In 1985, Brown & Goldstein were awarded the Nobel Prize for discovering the LDL receptor, which brings LDL into individual cells. Shortly after making this discovery and thinking that normal LDL initiated atherosclerosis via the creation of a foam cell, they conducted an experiment.
Foam cells are macrophages, a type of immune cell, that look foamy after they've engulfed the fat and cholesterol molecules removed from the artery wall.
Atherosclerosis, the deposition of plaques of fatty material on the inner artery walls, is one of the things thought to lead to heart attacks (i.e., myocardial infarctions). So, the researchers incubated macrophages with LDL particles to see if LDL alone would stimulate atherosclerosis.
To their surprise, the macrophages did not become foam cells. Only after undergoing “modification” would LDL particles induce macrophages to become foam cells [*].It was something about the quality of that LDL particle and its contents that initiated the first step in atherosclerosis.
Later, other scientists discovered the nature of this modification: LDL oxidation occurs when omega-6 fatty acids in LDL oxidize to form primary oxidation products (like lipid hydroperoxides) and secondary oxidation products. Once sufficiently oxidized, these LDL particles are no longer recognized by LDL receptors but are instead recognized by scavenger receptors on cells, including macrophages. This leads to foam cell formation [*,*].
Linoleic acid is the primary fatty acid to oxidize in LDL particles [*,*]. The blood from patients with cardiovascular disease contains higher concentrations of linoleic acid [*]. And those with the most atherosclerosis have the highest levels of serum linoleic acid [*].
Thus “modified,” these particles of LDL are known as “oxidized” LDL or oxLDL for short [*,*,*].
LDL is often referred to as "bad cholesterol;" however, LDL isn't inherently harmful. Instead, the danger comes from the oxidation of linoleic acid in the LDL particles [*,*]. In other words, it's not just the oxidation of LDL; it's the oxidation of linoleic acid in LDL particles that may contribute significantly to heart disease.
Hermann Esterbauer was a pioneer in the study of lipids and their downstream products called metabolites. He emphasized the importance of a balance between the amount of oxidation-prone PUFAs like linoleic acid in LDL particles and their antioxidant content [*].
Esterbauer also identified one of the most harmful linoleic acid oxidation products, 4-hydroxynonenal (HNE).
HNE is a secondary peroxidation product of linoleic acid. This means that linoleic acid gets converted to a molecule, which in turn becomes HNE. This process is called lipid peroxidation. HNE is amongst the most toxic breakdown products to living cells. It causes pathology in multiple diseases, depending on its concentration and the tissue in which it is acting.
HNE, along with other aldehydes like MDA, 4-ONE, acrolein [*], and the manifold OXLAMs (oxidized linoleic acid metabolites), are now considered to be involved in the initiation and progression of heart disease. Such work into the modification of LDL introduced the idea that the quality of LDL was more important than absolute amounts of LDL in heart disease into the mainstream of cardiology.
For example, oxidized LDL levels above the normal range in healthy men are associated with a mild increase in relative risk of cardiovascular disease on the order of 17-23% [*,*]. In people 60 or younger, however, those with a higher proportion of oxidized LDL particles are at a 16.8-fold higher risk of coronary artery disease [*]. That’s on par with the increased risk of lung cancer from smoking [*].
A recent estimate suggests that when corrected for a common form of oxidized LDL known as “lipoprotein (a)” — abbreviated Lp(a) — LDL cholesterol alone does not impact heart disease risk [*].
It was always doubtful that LDL particles alone would be inherently atherogenic. The hypothesis, colloquially referred to as the “clogged pipe” model, proposed that a high concentration gradient of LDL particles building up in the bloodstream would, somehow, forcefully invade the arterial wall [*].
However, arterial endothelial cells masterfully decide what to let in or keep out. They keep out water and even single ions (i.e. atoms) thanks to “tight junctions” that bind cells together to maintain tissue impermeability.
So keeping out LDL particles — which by analogy are the size of supertankers (8-25 nm) compared to row boat-sized water molecules (0.27 nm) — is no problem. Consequently, a qualitative change, like the oxidation levels in LDL particles or the integrity of the arterial wall, can better account for how LDL particles end up in atherosclerotic arteries.
Numerous human clinical studies also show that increased seed oil consumption also increases LDL's susceptibility to oxidation [*,*,*,*,*]. This is because LDL lipoproteins contain OXLAMs, such as the aldehydes mentioned above. These OXLAMs cause the abnormal accumulation of lipids and the recruitment of white blood cells, which form foam cells, a key feature of atherosclerosis [*].
A recent review of the causation of heart disease proposes that the first step in the disease process is the oxidation of the unsaturated fats and cholesterol esters in LDL [*].

Study figure 5. When sufficient OXLAMs are present in LDL lipoproteins they induce lipid accumulation, the recruitment of immune cells, and the eventual formation of foam cells, a key feature of atherosclerosis [*].
So, how can someone reduce their levels of oxidized linoleic acid in LDL particles?
The two primary ways would be to reduce or eliminate high linoleic acid seed oil consumption and to avoid smoking cigarettes [*].
It’s important to note that during the extraction, processing, storage, and cooking of seed oils, they become somewhat oxidized and thus contribute further to arterial damage [*]. In summary, the oxidation of linoleic acid is a major concern when it comes to cardiovascular disease. The very definition of oxidized LDL is LDL with oxidized linoleic acid, and this is one of our best markers for heart disease risk.
But What About Cholesterol?
Cholesterol is trafficked around the body largely in the form of VLDL (very low-density lipoprotein) and LDL particles, returning to the liver via HDL (high-density lipoprotein) particles [*]. Cholesterol is not incorporated into these particles in the free form but is bound (esterified) to a fat molecule.
The most common fats incorporated into cholesterol esters are oleic acid and linoleic acid [*]. Linoleic acid, being unstable, degrades to multiple peroxidation products such as 9- and 13-HODE, which are commonly found in atherosclerotic plaques bound to oxidized cholesterol. OXLAMs are found in increasing numbers with age or as atherosclerotic disease progresses, and in the highest amounts after a heart attack [*].
Cholesterol is also affected by the oxidation of linoleic acid. Oxidized linoleic acid drives the oxidation of cholesterol into toxic oxysterols, such as 7-ketocholesterol [*]. Oxysterols and oxidized linoleic acid are both found in high amounts in oxidized LDL and drive atherosclerosis in animal models. You’ll also find the production of thromboxane, a hormone released from blood platelets, which induces excessive clotting.
“These results suggested that oxysterols, not cholesterol, were responsible for injury to human arterial smooth muscle cells” [*].
Oxidized LDL contains vastly more oxysterols than normal cholesterol, due to the oxidative burden imposed by linoleic acid.
Seed Oils as a Driver of Oxidative Stress, Inflammation, Coagulation, and Thrombosis
One way to think of atherosclerosis is the repeated process of injury and incomplete repair of arteries. These injuries could come from a variety of factors, like lead and air pollution [*,*]. Clotting disorders are another factor, such as sickle cell disease, which injures arteries and greatly increases the risk of cardiovascular disease [*,*,*,*]. Infection is also associated with atherosclerosis [*,*] and acute coronary syndromes such as myocardial infarction [*,*], although not in populations eating a non-industrial diet void of seed oils [*].
The common factor these processes share is what is known as oxidative stress. Oxidative stress is characterized by low antioxidant levels in the body in comparison to reactive oxygen species, also known as free radicals. When you have more free radicals than antioxidant defenses, you’re under oxidative stress.
Seed oils, largely via their high susceptibility to oxidation, greatly increase oxidative stress, and thus increase arterial damage [*]. Structures such as LDL damaged by oxidized linoleic acid directly induce inflammation in multiple diseases [*]. In fact, products of omega-6 oxidation are often used as markers of oxidative stress [*]. Inflammation-absent infection is known as “sterile inflammation,” and the markers used to identify it are typically OXLAMs [*,*].
So, seed oil intake drives inflammation, oxidative stress, and the dysfunction of cells lining the inner arteries (called endothelial cells) [*]. These three processes are crucial to the development of atherosclerosis.
Despite lowering cholesterol levels, consuming a diet of 15% of calories from omega-6 fats significantly increases markers of oxidative stress in humans, compared to a diet of 5% calories from omega-6 fats (the vast majority of which is linoleic acid) [*]. And multiple studies have shown that increasing omega-6 fats without addressing omega-3 intake (similar to what Americans actually eat) worsens heart disease outcomes and mortality [*].
So, linoleic acid induces an inflammatory environment in human endothelial cells [*] and its oxidized metabolites (OXLAMs) can increase blood clots and constrict blood vessels [*,*,*,*]. High linoleic acid intake can also increase the conversion of arachidonic acid to proinflammatory factors called eicosanoids, driving up inflammation even further [*,*].
Luckily, there’s evidence that lowering linoleic acid consumption reduces OXLAMs and decreases the concentration of linoleic acid in cells.
In a study called the MARGARIN study, a protein called CRP, which is released by the liver during inflammatory events, rose slightly in the group eating more linoleic acid over two years [*,*]. Fibrinogen levels, a marker of increased blood coagulability (or clotting), significantly increased in humans after consuming linoleic acid-enriched margarine [*,*].
Moreover, sunflower oil with an omega-6/3 ratio of 28/1 can increase platelet aggregation compared to a low omega-6/3 ratio seed oil [*]. In fact, elevated coagulation is a superior risk factor compared to elevated LDL cholesterol or particle levels [*]. And, as noted above, oxidized LDL directly induces increased production of clotting elements thromboxane leading to increased coagulation [*].
Compared to linoleic acid, alpha-linolenic acid (ALA) reduces inflammation, such as CRP, serum amyloid A, interleukin-6 (IL-6), and IL-6 receptor levels [*,*].
In animal studies, linoleic acid increases several inflammatory markers [*], and replacing linoleic acid-rich safflower oil with high omega-3 flaxseed oil reduces atherosclerosis and the amount of newly formed blood clots in mice [*]. In mice, the lowest omega-6/3 ratio diet led to less atherosclerosis and less severe atherosclerosis, whereas the severity of atherosclerosis increased when the omega-6/3 ratio did as well [*].
In other words, seed oil consumption has been shown to drive inflammation, oxidative stress, thickening of the blood, and cardiovascular disease processes more broadly in both human and animal models [*].
It’s important to keep in mind that clotting is meant to repair and protect arteries. Nonetheless, arteries end up accumulating damage when chronically in need of repair. These processes are associated with an even larger issue called metabolic syndrome, which itself is strongly linked to heart disease.
Seed Oils and Metabolic Syndrome
The late great pathologist Dr. Joseph Kraft performed thousands of autopsies on his patients to whom he had previously administered an oral glucose tolerance test (OGTT) with insulin. This test assesses one’s degree of insulin resistance, the key feature of diabetes. It uncovers diabetes earlier than the conventional criteria using elevated fasting plasma glucose.
His key finding was that “Those with cardiovascular disease not identified with diabetes are simply undiagnosed [diabetics] [*,*].” That is to say — although his patients with visible heart disease (upon autopsy) didn’t necessarily have elevated fasting plasma glucose levels, they were always diabetic according to better criteria, namely an OGTT with insulin.
Diabetes is, in essence, an advanced state of metabolic syndrome [*]. The latter is diagnosed by having at least three of five pathological markers:
Abdominal adiposity
Low HDL cholesterol
High triglycerides
Hypertension
Elevated fasting plasma glucose levels
You could also add in elevated fasting insulin as a sixth marker, since every stepwise increase in fasting insulin — technically, one standard deviation —increases your odds of cardiovascular disease by 60% [*]. Fasting insulin elevates as a compensatory response to cells resisting the action of insulin. Reducing oxidized LDL via antibody therapy reduces insulin resistance in a primate model [*]. Insulin itself induces cells to increase uptake of oxidized LDL, quickening the process of atherosclerosis via induction of macrophages to become foam cells [*].
Seed oils can induce most, if not all, of these pathological markers. In the short-term, they do this by pushing fat cells towards their maximal expansion capacity — more so than other more saturated fat sources [*,*]. As this progresses over the long term, fat cells get closer and closer to their expansion limits. Fatty acids then start leaking out inappropriately [*]. This increases fasting blood levels of triglycerides, glucose, and eventually insulin. A person is then diagnosed with metabolic syndrome.
And metabolic syndrome is associated with a 288% increased risk of heart disease [*].
Mechanisms Implicating Seed Oils as a Causal Factor in Heart Disease
There’s abundant evidence implicating high linoleic acid seed oils as a causal factor in cardiovascular disease, starting with test tube (in vitro) experiments and ending with large multi-year randomized-controlled trials (RCTs). Consilience of evidence, or having different lines of evidence pointing in the same direction, increases confidence in this hypothesis.
When researchers expose human umbilical cord cells to common (physiological) concentration of linoleic acid, it spurs a proinflammatory environment [*]. When exposed to linoleic acid, human aortic endothelial cells produce an abundance of reactive oxygen species (ROS), inflammatory factors, and adhesion molecules — all of which are required for the atherosclerotic process [*,*]. Cells from pigs, specifically vascular endothelial cells, react with an inflammatory cascade when exposed to linoleic acid [*,*].
Heart cells from rats exposed to linoleic acid metabolites had abnormal electrical currents, suggesting that linoleic acid increases the risk of abnormal heart rhythms (arrhythmias) and cardiac arrest [*]. Dogs suffer cardiac arrest from a metabolite of linoleic acid called EOA (aka leukotoxin), which acts like a bacterial toxin in their hearts. The authors of this dog study point out that “linoleic acid metabolites have been shown to be very toxic in a variety of cell types, in several species of animals, and most importantly in humans [including] cell death [...] and multiple organ failure.” Leukotoxin has also been implicated in severe disease in COVID-19 patients:
“This finding raises the possibility that amelioration of COVID-19 symptoms may be achieved in part by reduction of omega-6-rich diet, or an enhanced omega-3 fatty acid intake in patients hospitalized for COVID-19” [*].
However, it’s not just linoleic acid that can be cardiotoxic — OXLAMs can be as well. And although OXLAMs can provide a useful danger signal for cells to protect themselves, they are also known to recruit white blood cells to injured tissue, which is crucial to the atherosclerotic process [*,*,*].
In a mouse model that’s genetically modified to be more susceptible to heart disease, a 1:1 tissue ratio of linoleic acid to omega-3 fatty acids led to fewer atherosclerotic injuries (lesions) compared to mice with higher ratios [*]. In mice given fish oil (low in linoleic acid and rich in omega-3), corn oil (rich in linoleic acid and low in omega-3), or a lower-fat control diet to eat freely, the fish oil group had less atherosclerotic plaque [*].
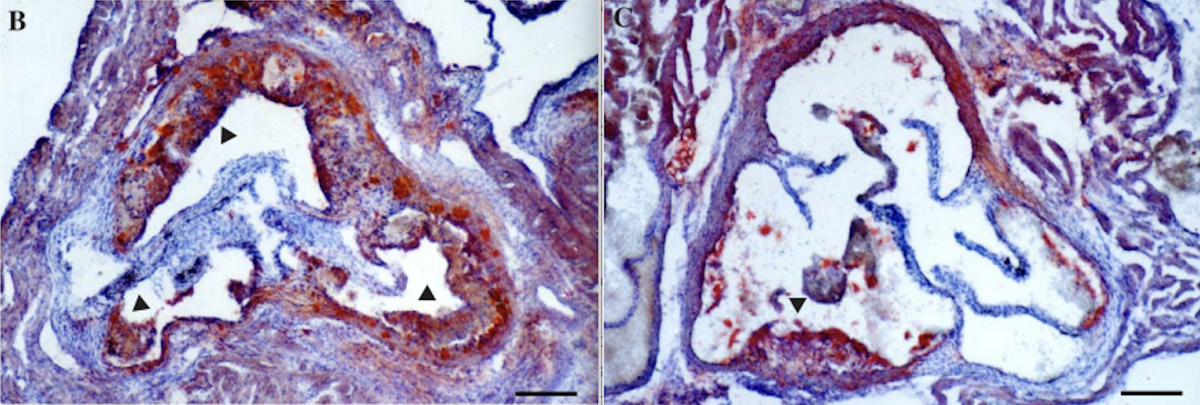
Study figure 1. Panel B shows a cross-section of mice arteries fed corn oil and panel C fish oil. The more red staining, the worse the atherosclerosis [*].
Human autopsies can provide a lot of granular detail on disease processes as well. They reveal that linoleic acid is the most abundant fatty acid in human atherosclerotic plaque [*]. The more severe the patient’s heart disease was before their death, the more oxidized linoleic acid was found in their plaque [*]. In comparison, people killed in car accidents have less linoleic acid in their arterial plaque than those who die from a heart attack [*]. This is seen in other mammals as mentioned in the previous section, where mice with more severe heart disease have a higher omega-6 to omega-3 ratio in their plaque.
These observations about linoleic acid levels and heart disease — both in cell and animal experiments — are only correlations and mechanistic explanations. High-quality human RCTs show causation.
In one RCT, groups of veterans were fed different amounts of linoleic acid over a span of 8 years. After their deaths, researchers found that the group eating the most linoleic acid had more linoleic acid in their fat tissues and platelets (which serve to clot blood), as well as more atherosclerosis [*].
Another RCT gave patients fish oil or sunflower oil capsules (but no placebo) before they underwent surgery to remove arterial plaque [*]. Compared to the fish oil group, the sunflower oil group had more plaque in their arteries and higher rates of plaque ruptures.
Although many human trials support the idea that seed oils are directly implicated in heart disease, some don't. But the devil is in the details, so to determine which studies have it right, we must dive into their methodology.
Mistakes That Led to the “Heart Healthy” Myth
The scientific study of dietary fats on health has been contentious since the days of the cholesterol and dietary fat hypothesis of heart disease. First, associations between lower blood levels of linoleic acid and an increased risk of heart disease have misled researchers into recommending that people consume more dietary linoleic acid to reduce their risk.
Second, the view of atherosclerosis essentially being a process of LDL particles invading the artery from “the inside-out” — like fat clogging up a pipe — has proven incorrect, yet remains foundational to the field.
Third, there have been key studies with major flaws published on the topic that are important to know about.
Understanding these three contentious points helps clarify why linoleic acid has come to be seen as “heart healthy” despite so much evidence that proves otherwise.
Confusing Associations Between Blood Levels Of Linoleic Acid And Heart Disease Risk
We can set the stage for some of these confusing associations by reviewing some basic physiology. Approximately quadrupling your intake of dietary linoleic acid from 5% to 21% of total calories increases blood plasma linoleic acid (as a percentage of total fatty acids) by 19.4% [*]. This translates to an increase from 9% to 10.8% linoleic acid as a percentage of total blood plasma fatty acids. Simply put, the more linoleic acid you eat, the more of it ends up in your blood.
As we’ve seen, higher linoleic acid plasma levels can increase your risk of heart disease. So, how is it that lower levels of linoleic acid in the blood have also been associated with an increased risk of heart disease and death [*,*,*]?
These findings are likely due to something called reverse causation. Reverse causation occurs when another factor that increases the risk of heart disease also lowers linoleic acid in the body, making it seem as if having low linoleic acid levels in the body is bad.
Enzymes such as D6D can increase downstream metabolites of linoleic acid in blood plasma and thus levels of the fat, leading to more inflammatory precursors [*]. Higher inflammation correlates with less linoleic acid levels in the blood [*,*], and increased levels of OxLAMs are associated with disease progression.
There are two takeaways here. First, that the relationship between dietary intake of linoleic acid and blood plasma levels of the fat are context dependent. Second and most importantly, it’s not just the intake of dietary linoleic acid that’s a problem; the oxidation of linoleic acid is the most significant issue [*]. Lower plasma linoleic acid levels are a result of, not a cause of, a process leading to heart disease.
When we look at a more comprehensive set of biomarkers for linoleic acid intake, there is an increased severity of coronary artery disease in people with high levels of linoleic acid in fat tissue and in blood clotting factors called platelets [*].
Additionally, angiograms, or scans that show blood flow through arteries or veins, show that an increased intake of linoleic acid is associated with an increased risk of new atherosclerotic lesions in human coronary arteries [*].
So, although lower levels of linoleic acid in the blood have been associated with an increased risk of heart disease, a high dietary intake of linoleic acid (confirmed with samples from fat tissue and platelets) is associated with an increased risk of heart disease.
The “Clogged Pipe” Model Of Atherosclerosis Is Wrong
We previously mentioned the “clogged pipe” model of how atherosclerosis develops. It proposes that LDL particles invade the artery via the lumen, which is the space where blood flows through the artery [*].

The Encyclopedia Britannica’s depiction of the mainstream model of atherosclerosis. LDL particles invade the artery via the lumen (i.e., the inside the space where blood flows) [*].
But this model fails to account for the artery being impermeable but highly selective of what it lets through. LDL particles don’t force their way in, even when in high concentrations in the blood.
One prediction of luminal invasion is that the highest concentration of LDL particles will be found in the intima (labeled “I” below), the first part of the artery starting from the lumen. And the further away from the intima — towards what’s called the media (labeled “M”) — the lower the concentration will get. You would expect to see a gradient pattern of lipid deposition [*].

Study figure 2. A real cross-sectional picture of an artery showing the intima (I) and the media (M). A drawing of an artery color-coded to show the inner intima, outer intima, inner media and outer media [*].
Autopsies reveal something quite different, if not the opposite — the inner and outer intima have fewer LDL particles than the media.
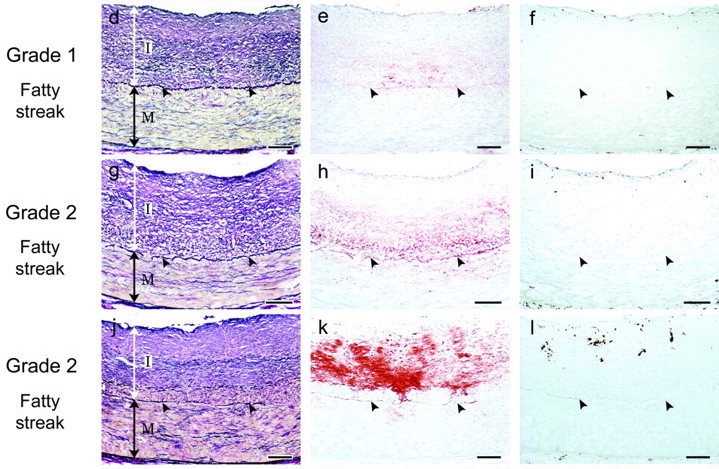
Study figure 3. Red staining represents LDL particles in the intima (I) and media (M). There’s no gradient pattern of lipid deposition with higher concentration in the intima and a lower one in the media [*].
How atherosclerosis actually develops is not known. Whatever the correct explanation turns out to be, the real gradient pattern of lipid deposition is not compatible with the “clogged pipe” model of atherosclerosis. Without it, there is no basis upon which to recommend that people consume seed oils to lower the concentration of LDL particles in their blood.
How Category Errors Assigned Benefits From Omega-3 To Linoleic Acid
Many early studies on heart disease also contained a design flaw called a category error.
Studies would often conflate linoleic acid with the omega-3 docosahexaenoic acid (DHA) and ALA because they’re all PUFAs. Combining these fats and analyzing the results of PUFA consumption is inappropriate when seeking causative effects of linoleic acid consumption.
Different PUFAs not only have distinct roles but can act in opposite ways. For example, as we’ve discussed, linoleic acid is generally a precursor to inflammatory compounds, while DHA and ALA are generally precursors to anti-inflammatory ones.
This category error can quite literally invert results, as revealed by meta-analyses of human trials. When only trials conflating linoleic acid with DHA and ALA are considered, linoleic acid appears to have no effect or even a beneficial effect on heart disease.
When only the trials that do not make this category error are considered, linoleic acid’s harms are revealed as having “increased the risks of all relevant causes of coronary heart disease outcomes [*].” Unfortunately, a high intake of linoleic acid and a low intake of omega-3 fats typifies the modern industrial diet.
Studies That Support Seed Oils as “Heart-Healthy”
As you can see, evidence is clearly mounting against seed oils as a “hearty-healthy” choice. But what about the studies that do seem to support the consumption of high linoleic acid oils? Let’s take a look.
One study that is used to support the consumption of seed oils is the Finnish Mental Hospital Study. In the study, subjects who consumed more seed oils had a lower risk of death from heart disease.
However, there were major flaws with the study design. For example, the consumption of artificial trans-fat was also removed from the “omega-6 intervention.” In other words, it wasn’t just a higher intake of omega-6 fat that occurred in this group but also a lower intake of industrial trans fats, which are a major known contributor to heart disease.
Therefore, it’s impossible to conclude whether it was the lower intake of trans fat or the higher intake of linoleic acid that led to the results. Other flaws of the study include (1) no randomization and (2) unequal between-group baseline differences for blood pressure, cigarette smoking, and the use of certain cardiotoxic medications favoring the seed oil group. Thus, the Finnish Mental Hospital Study cannot provide valid evidence for or against consuming seed oils.
Another study that’s used to support the intake of seed oils is the Los Angeles Veterans Administration study [*]. This study was a multi-year randomized, double-blind trial in 800 male veterans with and without previously diagnosed coronary heart disease.
The trial aimed to find out what would happen as a result of diets higher in seed oils. Despite the seed oils group dropping their total cholesterol nearly 13% lower than the control group, there was no significant impact on heart attacks or sudden death.
Major flaws in this study include unequal between-group baseline differences in cigarette smoking and “probable” heart attacks, both of which degrade the evidence completely.
Furthermore, the control group that was consuming lower levels of seed oils was “distinctly deficient” in vitamin E, which could have led to more deaths from heart disease. The seed oil group was also an “omega-3” group, as these individuals consumed ~ 7-fold more omega-3 compared to the control group (700 mg/day versus 100 mg/day, respectively). Like in the Finnish Mental Health Study, trans-fat intake was restricted in the seed oil group but was more than 2 grams per day in the group eating less seed oil.
So, the two main studies used to support seed oil consumption may be hopelessly flawed. The Los Angeles Veterans Administration study may just as well support a reduction in trans fats and an increase in omega-3 fats, both of which are already well-established beneficial dietary interventions.
Studies That Show Seed Oils Are Harmful
While the two major “randomized” trials showing benefits from seed oils are fatally flawed, the randomized trials showing their harms, while not perfect, are more rigorous.
For example, The Minnesota Coronary Survey was a randomized, double-blind clinical trial in over 9,000 men and women [*]. This study compared a control diet with 5% omega-6 PUFAs to one with three times as much (mainly from trans fat-laden margarine and seed oils). Those who were assigned to the 15% omega-6 diet saw a reduction in their cholesterol levels.
However, the number of people who died in both groups was statistically indistinct after a few years. That said, the authors display caution about the long term trend revealing itself in the 15% omega-6 group, saying “The small difference between the two life tables is in an unfavorable direction.”
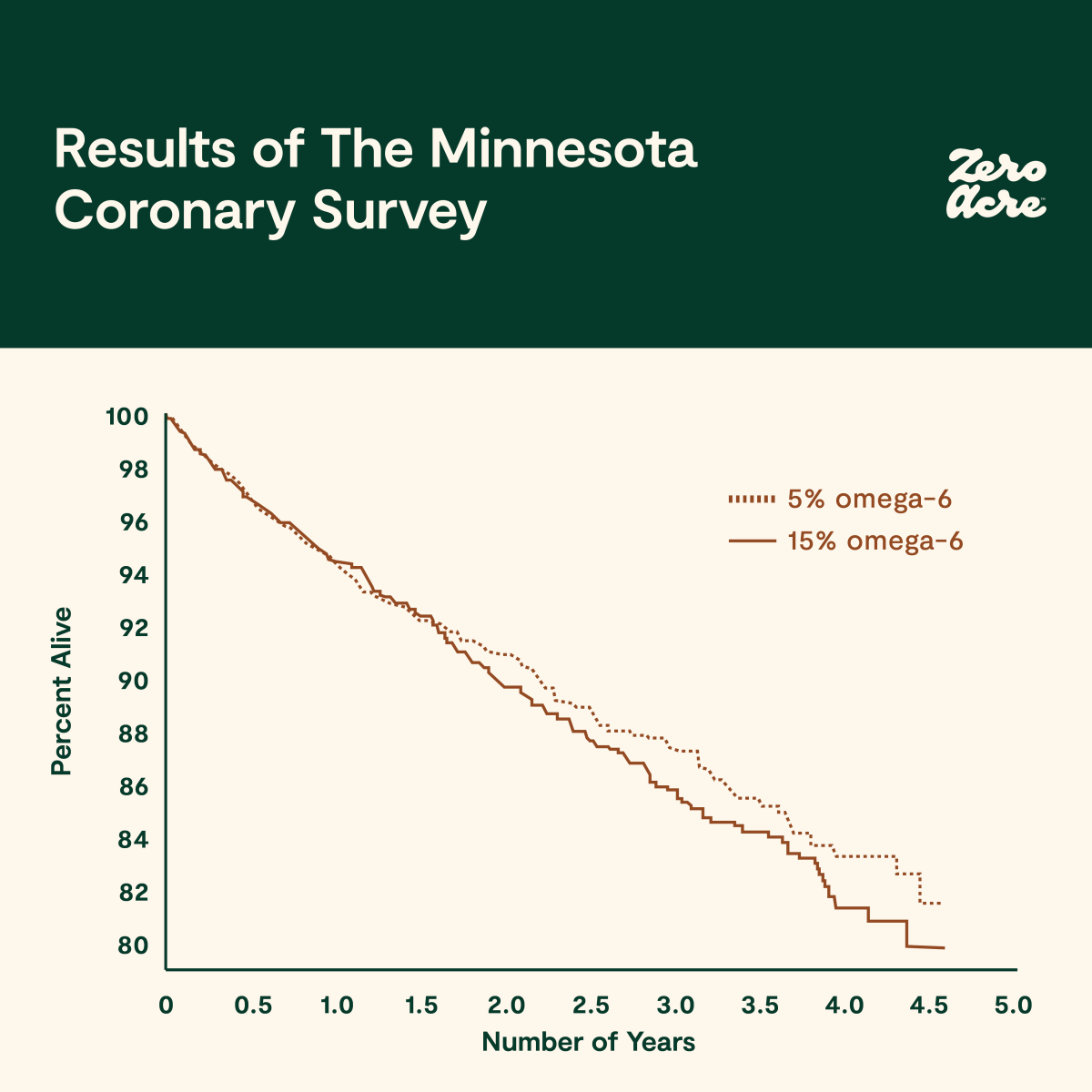
Study figure 6. The percentage of men and women alive in the treatment (—, 15% omega-6) and control groups (•••, 5% omega-6) over 5 years. [*].
For women, however, there was a relative risk increase in death from heart disease (+28%), non-fatal heart disease (+25%), and all-cause mortality (+17%). This study shows that consuming seed oils can lead to lower cholesterol levels, but still increase a woman’s relative risk of death from heart disease and all-causes.
A re-analysis of the study’s provisional autopsy data found that 41% of the seed oil group had at least one heart attack versus 22% of controls [*]. And although the re-analysis cannot verify the statistical significance of these results because of missing raw data, the apparent increase in all-cause mortality in the seed oil group appears confined to those aged 65 and above. Like smoking cigarettes and developing lung cancer, the deadly effects of seed oil consumption may take years or decades to develop.
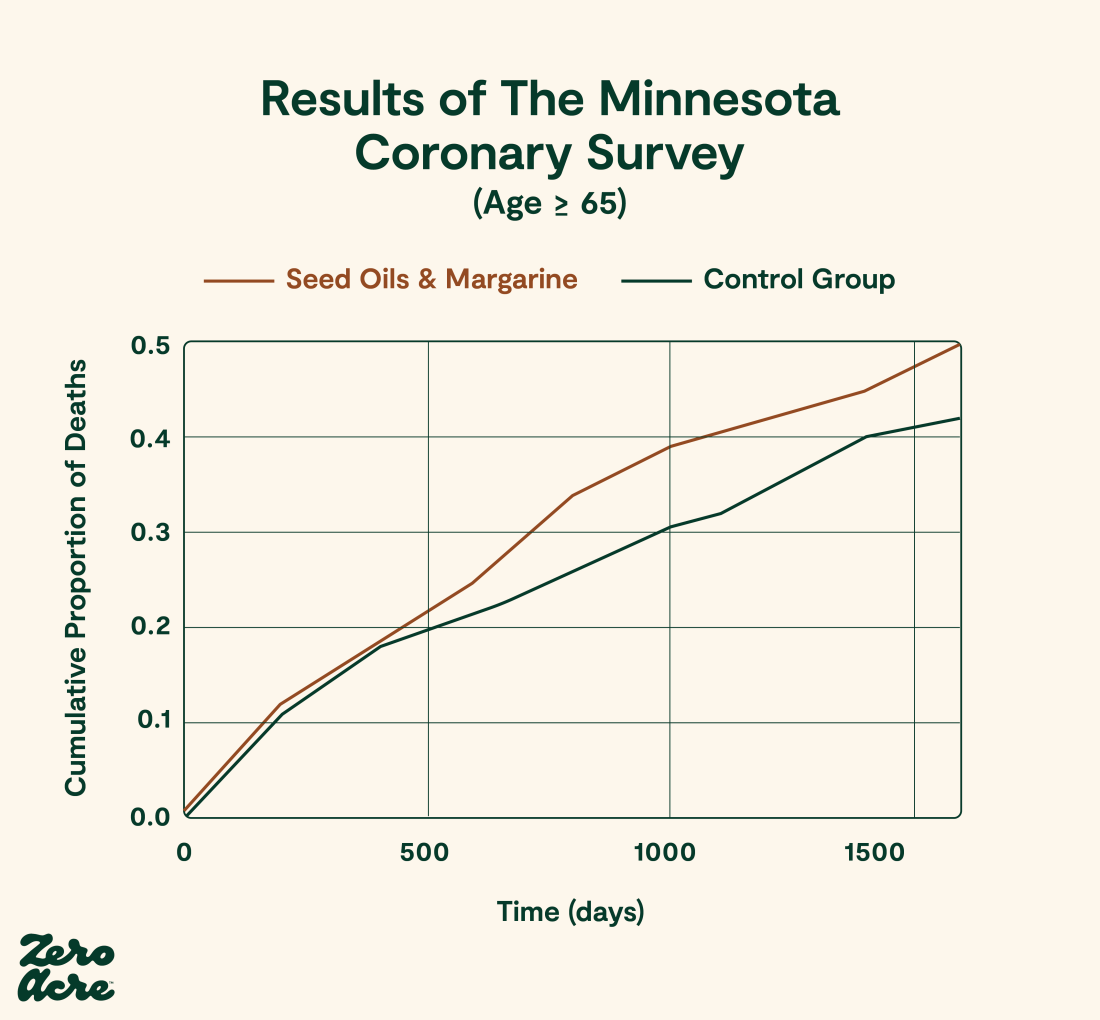
Study figure 5 reproduced by Jeff Nobbs [*]. A Kaplan Meier life table graph of cumulative death from any causes over 1,600 days in patients aged ≥ 65.
The Minnesota Coronary Survey highlights a robust relationship between lower total cholesterol levels and increased risk of death, even when accounting for people with lower cholesterol levels. It also doesn’t support the diet-heart hypothesis, which postulates that replacing saturated fat with omega-6 PUFA lowers cardiovascular mortality and all-cause mortality.
The 1965 Rose Corn Oil study randomized 80 patients with coronary heart disease to either a control diet, a diet restricted in saturated fat and supplemented with refined olive oil, or a diet restricted in saturated fat and supplemented with corn oil [*].
The mean intake in the olive oil group was 58 g/day and 64 g/day in the corn oil group. Assuming the corn oil had a 60% linoleic acid concentration — the paper didn’t specify — this means subjects consumed just under 40 grams of linoleic acid per day from corn oil. At the end of the study, the proportions of patients that were alive and free of a fatal or non-fatal second heart attack were 75%, 57%, and 52% in the control, refined olive oil, and corn oil groups, respectively. In other words, those consuming the corn oil fared the worst.
The Sydney Diet Heart Study was a randomized controlled trial conducted from 1966 to 1973. It tested the effects of a higher linoleic acid intake from safflower oil (about 75% linoleic acid) replacing saturated fats from animal fats, margarines, and shortenings, against a control group receiving no specific dietary instructions [*]. Researchers found that 17.6% of the safflower oil group died versus 11.8% of the control group, which is equivalent to a 62% increase in the relative risk of death (p = 0.051) [*]. And 17.2% of the safflower oil group died of cardiovascular disease versus 11% in the control group, amounting to a 70% increase in the relative risk of death from cardiovascular disease (p = 0.04).
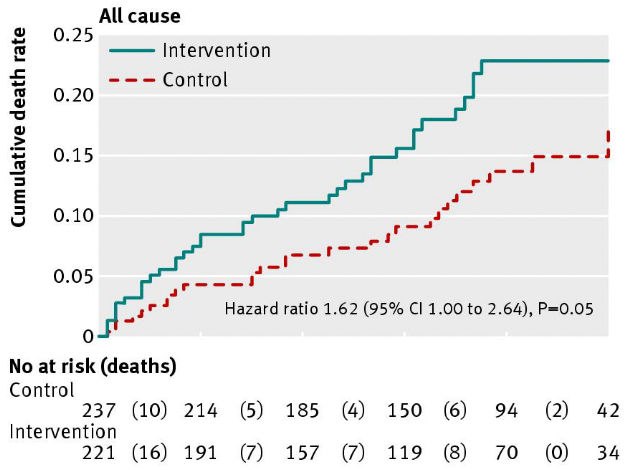

Study figure 2 showing the progression and cumulative all-cause and cardiovascular disease mortality over 5 years in the Sydney Diet Heart Study [*].
Notably, total cholesterol decreased more in the safflower group than in controls (-13.3% versus -5.5%). And a re-analysis of the Sydney Diet Heart Study found that the safflower oil group had a 17.2% increase in the relative risk of death from cardiovascular disease, specifically.
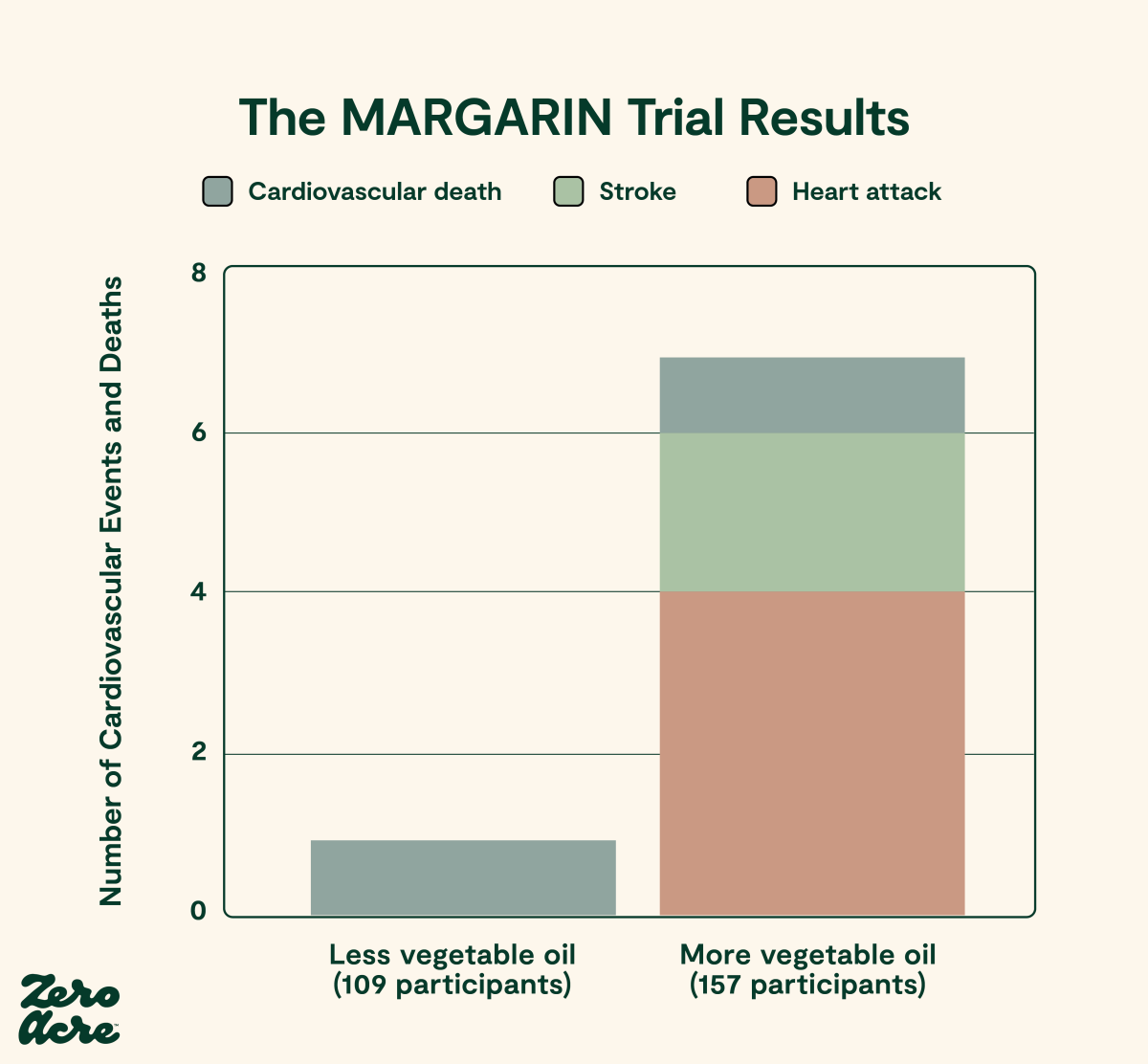
The MARGARIN trial was a multifactorial intervention about diet and nutrition education, so it cannot isolate effects from linoleic acid [*]. It consisted of four groups that were double-blind but not randomized and compared people who were educated about a Mediterranean-style diet and people who were simply given the Dutch Dietary Guidelines leaflet. Both groups received margarine that was either 58% linoleic acid and 15% omega-3 from ALA, or 46% linoleic acid and 0.3% ALA.
The authors report no statistically significant differences between groups in terms of the 10-year risk of suffering a cardiovascular event (either a cardiovascular death, heart attack, or stroke). However, there is a signal favoring ALA when combining both ALA-rich margarine groups and comparing it to the combined linoleic acid-rich margarine one: there’s one cardiovascular event in the former versus seven in the latter.
A smaller RCT recommended a Mediterranean diet or a low-fat diet (the control) for 5 years to patients who’d already suffered from a heart attack [*]. The Mediterranean diet consisted of multiple dietary changes, including a higher ratio of polyunsaturated to saturated fat closer to 1 (i.e., 0.73) than the control group (i.e. 0.59). After 5 years, the Mediterranean diet group reported 70% lower all-cause mortality relative to the control group.
Systematic Reviews and Meta-Analyses of Clinical Studies
One of the biggest reasons some believe seed oils are healthy is because of previous systematic reviews, which excluded the Sydney Diet Heart study and combined studies that increased both omega-3 and linoleic acid [*,*].
When studies that increased omega-3 intake were excluded, this ensured linoleic acid was the only variable affecting the risk of death. This more rigorous analysis reveals a 16% increase in the relative risk of death when linoleic acid replaces a combination of trans-fats and saturated fats, but fails to reach statistical significance (p = 0.146) [*]. Additionally, the risk of non-fatal heart attacks plus death from heart disease was higher in the linoleic acid trials compared to those combining omega-3 like ALA and linoleic acid (p = 0.02).
Meta-analyses are powerful tools in that they can compound scientific mistakes or help identify them. The latter occurred in a 2017 meta-analysis of randomized controlled trials replacing saturated fat with “mostly omega-6 polyunsaturated fat [*].” It was conducted with the explicit aim of not repeating the mistake of past meta-analyses that “did not sufficiently account for major confounding variables that were present in some of those trials.” Smoking, if not equally prevalent in the control and intervention group, is an example of a confounding variable. The author Steven Hamley concluded the following: “Available evidence from adequately controlled randomized controlled trials suggest replacing [saturated fat] with mostly omega-6 PUFAs is unlikely to reduce [coronary heart disease] events, [coronary heart disease] mortality or total mortality. The suggestion of benefits reported in earlier meta-analyses is due to the inclusion of inadequately controlled trials.”
The Major Confounders: Smoking and Treatment
As we know, the middle of the 20th century saw a major increase in rates of heart disease [*] and a significant increase in the consumption of seed oils. By the time the AHA recommended increased seed oil consumption in 1961, the U.S. had already seen a rise since the middle of the 19th century, when widespread adoption of cottonseed oil had begun. So, by the standard of prevention, recommending more seed oil consumption to prevent increased rates of heart disease was a failure the day it was proposed, never mind the trials that later were conducted showing it was harmful.
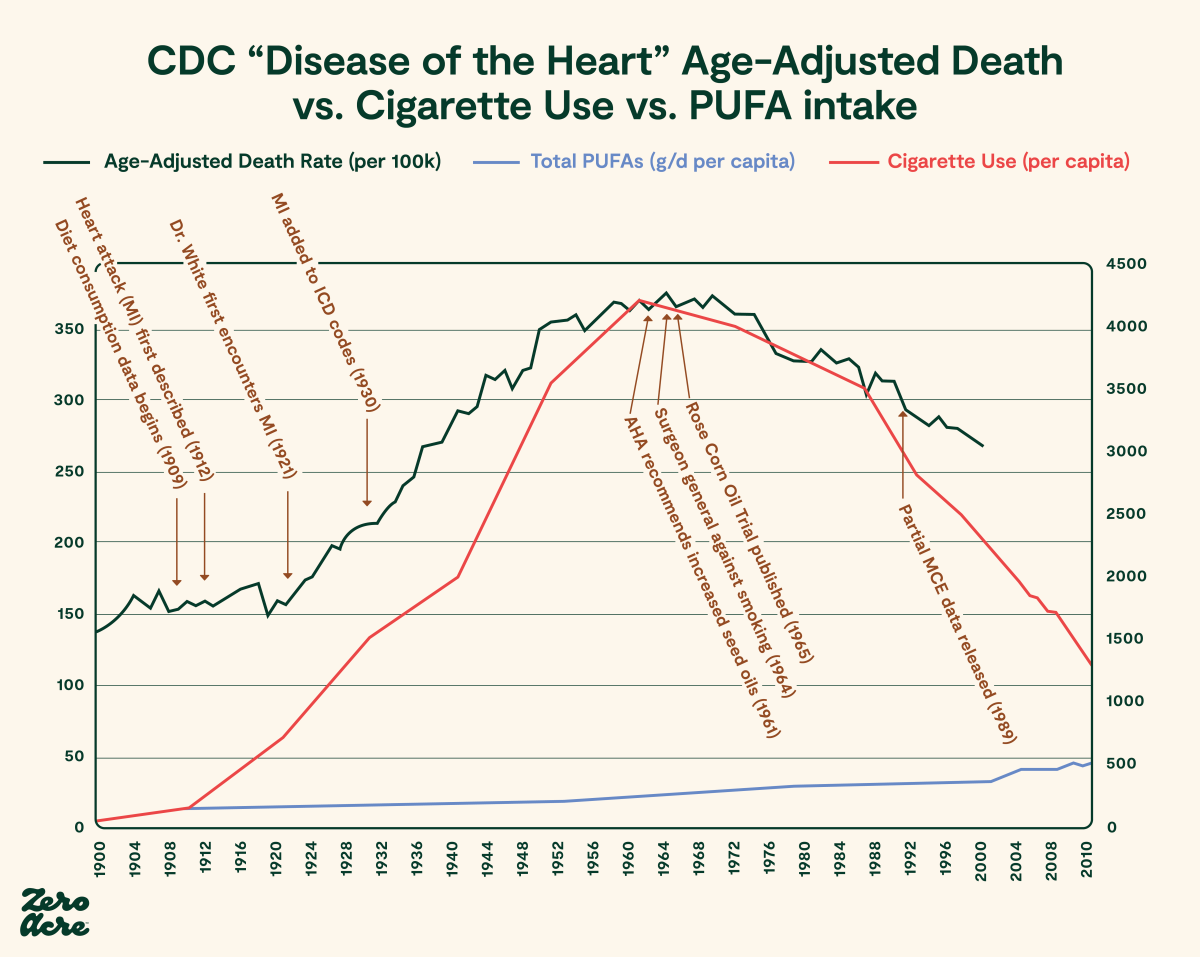
Of course, there is a major confounder: smoking. While the U.S. Surgeon general’s recommendation against smoking in 1964 was based on the association with lung cancer [*], it was later recognized that smoking also played a major role in heart disease [*].
Perhaps surprisingly, smoking and seed oil consumption share a common mechanism that cause heart disease. Smoking also induces oxidative stress [*], induces insulin resistance [*], damages the arterial endothelial wall [*], and increases oxidized LDL [*,*].
In fact, one of the major toxins in cigarette smoke, acrolein, is also produced from linoleic acid [*]. This commonality in mechanisms is reflected in the fact that frying with vegetable oils is the second major cause of lung cancer worldwide [*,*].
Smoking can also increase the amount of oxidized linoleic acid in the body [*]. Mouse experiments suggest toxins in cigarette smoke damage the heart’s cardiolipin, a crucial component of mitochondrial membranes. This is thought to occur when linoleic acid in these membrane structures oxidize, resulting in oxidative-stress-induced mitochondrial damage [*]. The latter symptoms lead to features of cardiovascular disease, like hypertension and reduced mitochondrial function, thus making oxidized LDL a risk factor for cardiovascular disease and likely a cause of atherosclerosis [*].
Ancestral populations such as the Kitavans or Tsimane didn’t consume industrially-produced seed oils, so they had less linoleic acid in their body to be oxidized by cigarette smoke, which could explain why smoking is not associated with heart disease in either of these populations [*,*]. This would also fit with the idea that decreased smoking rates in the United States, during which seed oil consumption was increasing, would have reversed the increase in heart disease. An additive effect of smoking and seed oil consumption may explain divergent disease prevalences in more industrialized countries as well, such as Japan [*,*].
At any rate, it is clear that smoking has a major effect on heart disease risk, and that the decline in heart disease rates since the Surgeon General’s report are in part due to a massive decline in smoking. However, heart disease rates in non-smokers in the U.S. are still much higher than rates in ancestral populations or in non-industrial countries in the period prior to the introduction of seed oils [*,*]. Heart disease remains the leading cause of death in the world, and certain aspects of heart disease, like heart failure, continue to increase [*].
Another major confounder for the decline in heart disease deaths in the U.S. is simply better treatment.
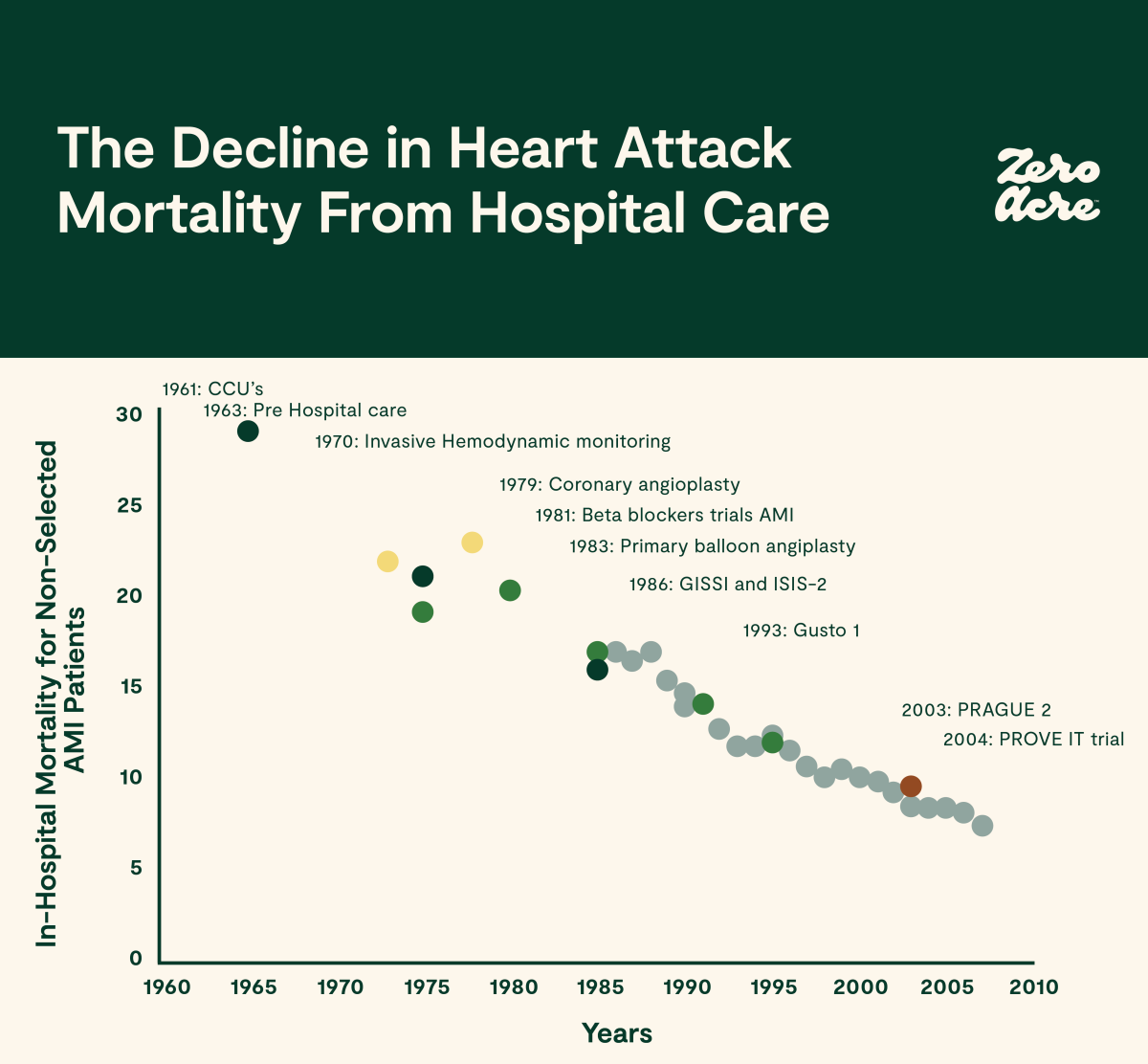
Medical treatment for heart attack (acute myocardial infarction or AMI) has steadily improved, both during an attack and afterward. Drugs such as statins, which affect the balance of oxidative stress in the body, may also play a role [*].
Unfortunately, untangling the early associations between seed oil consumption and heart disease is likely impossible in the U.S., as consumption of seed oils began in the 19th century, while data collection for the food supply (1909), mortality (1900-1933, by state), and heart disease (1912-1930) commenced much later.
Currently, we’re seeing increasing rates of heart disease in younger age groups in the U.S. who have often never smoked. This, combined with increasing heart disease risk factors such as obesity and diabetes [*,*], makes it clear that the steady increase in seed oil consumption continues to play a role in ongoing high rates of heart disease.
Conclusion
Of course, today, we have a much better understanding of the role seed oils may have played in President Eisenhower’s health and, subsequently, his death from advanced heart disease.
Linoleic acid is a chemically unstable fat with important signaling functions when consumed in evolutionarily appropriate amounts. The introduction of seed oils dramatically increased linoleic acid consumption, and this increase created a large burden of primary and secondary oxidation products, which are cardiotoxic to both humans and other animals.
Decades of human clinical studies looking at how different fats affect heart disease risk are rife with confounding variables and category errors. When these flaws are accounted for, the results flip from favoring linoleic acid to revealing a consistent signal of harm. This signal is all the more reliable given that all populations prior to the introduction of seed oils show low rates of heart disease. And once these pre-seed oil populations start consuming them, including the U.S. in the last hundred years, heart disease rates start to climb.
Given that increasing dietary linoleic acid above evolutionarily appropriate levels consistently increases heart disease mortality and all-cause mortality, one of the safest approaches to preventing heart disease may be to avoid seed oils.